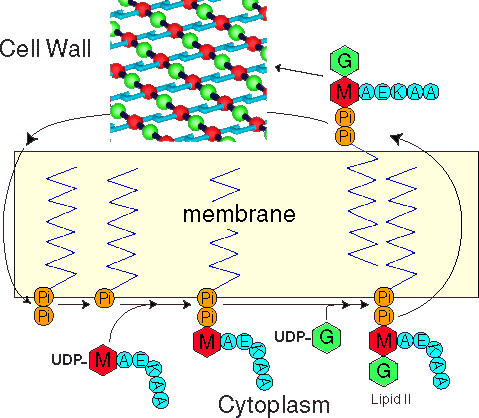
Uridine-diphosphate activated MurNAc-pentapeptide
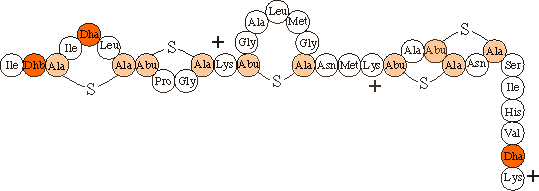
The lanthionine (Ala-S-Ala) and b-methyl-lanthionine (Abu-S-Ala) residues originate from the coupling of a cysteine to a dehydrated serine or threonine residue respectively.
Dephosphorilation. A step in the biosynthesis blocked by the antibiotic bacitracin.
Step catalysed by MraY
Timepoint of peptide addition
|
|
|
|
|
|
|
|
|
|
Nisin Page
(move your mouse pointer over interesting pieces to get more info)
|
|
|
|
 |
|
|
|
|
| under construction |
|
| Interaction of the lantibiotic nisin with Lipid II |
|
| The 34 amino acid sized peptide nisin is highly active against Gram-positive bacteria. This high activity is due to a combination of pore-formation and a high-affinity interaction with Lipid II. By gathering detailed information on the structural requirements for this interaction a blue-print for the development of new types of antibiotics can be developed. |
|
|
| The primary structure of nisin Z |
|
|
 |
|
| The Bacterial cell wall biosynthesis |
|
|
|
|
Its not a coinsidence that the bacterial cell wall synthesis in a popular target for antibiotics. An intact cell wall is essential for the viability of the bacteria. Thus, blockage of any step in the cell wall synthesis will eventually lead to death of the bacterial cell. The first antibiotic used by mankind, penicillin, works in such a way. Below you can see a movie on the action of penicillin on E. coli. |
|
|
|
|
|
|
| The role of Lipid II in bacterial cell wall biosynthesis |
|
|
 |
|
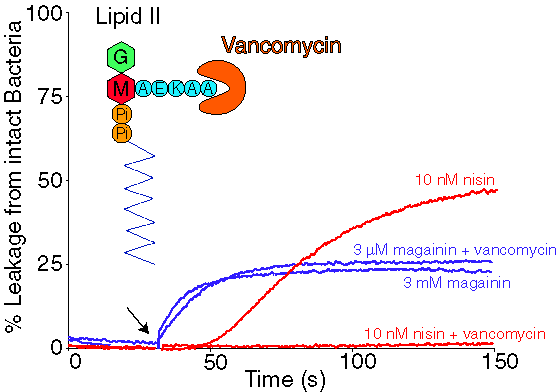
| The evidence that nisin interacts with Lipid II in vivo. |
|
|
|
It is generally accepted that vancomicin binds to the C-terminal D-Ala-D-Ala sequence of the pentapeptide of Lipid II. Thus, if vancomycin could inhibit nisin activity this would demonstrate that Lipid II is used by nisin in its antibacterial activity. The next figure demonstrates that vancomycin can inhibit the membrane permeabilisation activity (pore formation) of nisin, but not the activity of another antimicrobial peptide magainin (originating from frog-skin). |
|
 |
|
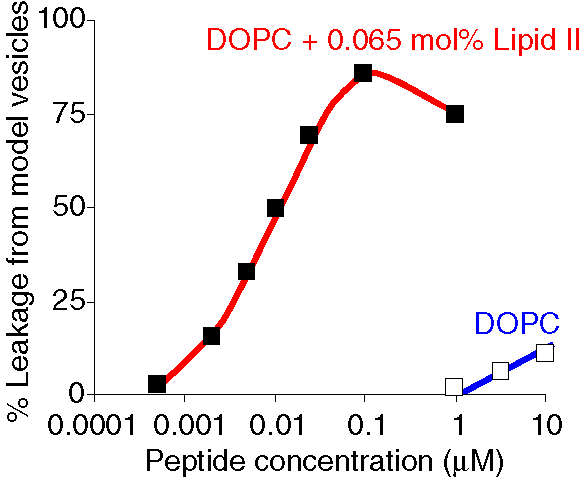
| Lipid II is the only target of nisin in the membrane. |
|
|
|
|
Adding purified Lipid II in small amounts (0.01-0.1 mol%) to model membrane vesicles dramatically increased the membrane permeabilisation activity of nisin (at least by a factor of 1000). The acticity of nisin in these systems is now in the same concentration range as the MIC values (minimal inhibitory concentration). This is a strong indication that Lipid II is the only target of nisin in the bacterial membrane. |
|
 |
|
|
|
|
Working model for the mode of action of the Lipid II mediated pore-formation by nisin |
|
|
Nisin initially interacts with Lipid II via its N-terminus. Then nisin assembles into a pore thereby inducing leakage of cytosolic contents. |
|
|
|
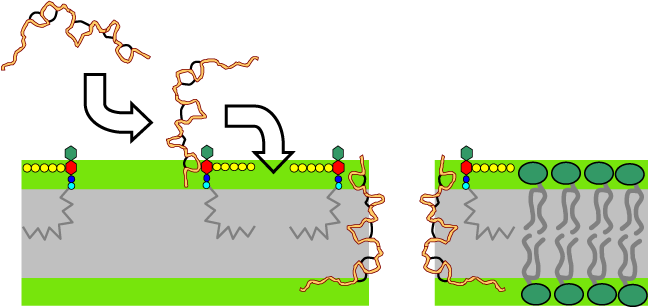 |
Click on arrowheads to go to the specific steps of the MOA. |
| The initial interaction of nisin with Lipid II |
|
| High affinity binding of nisin to Lipid II is mediated by the pyrophosphate cage |
|
|
|
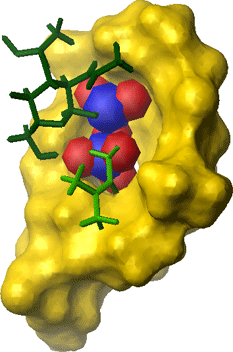
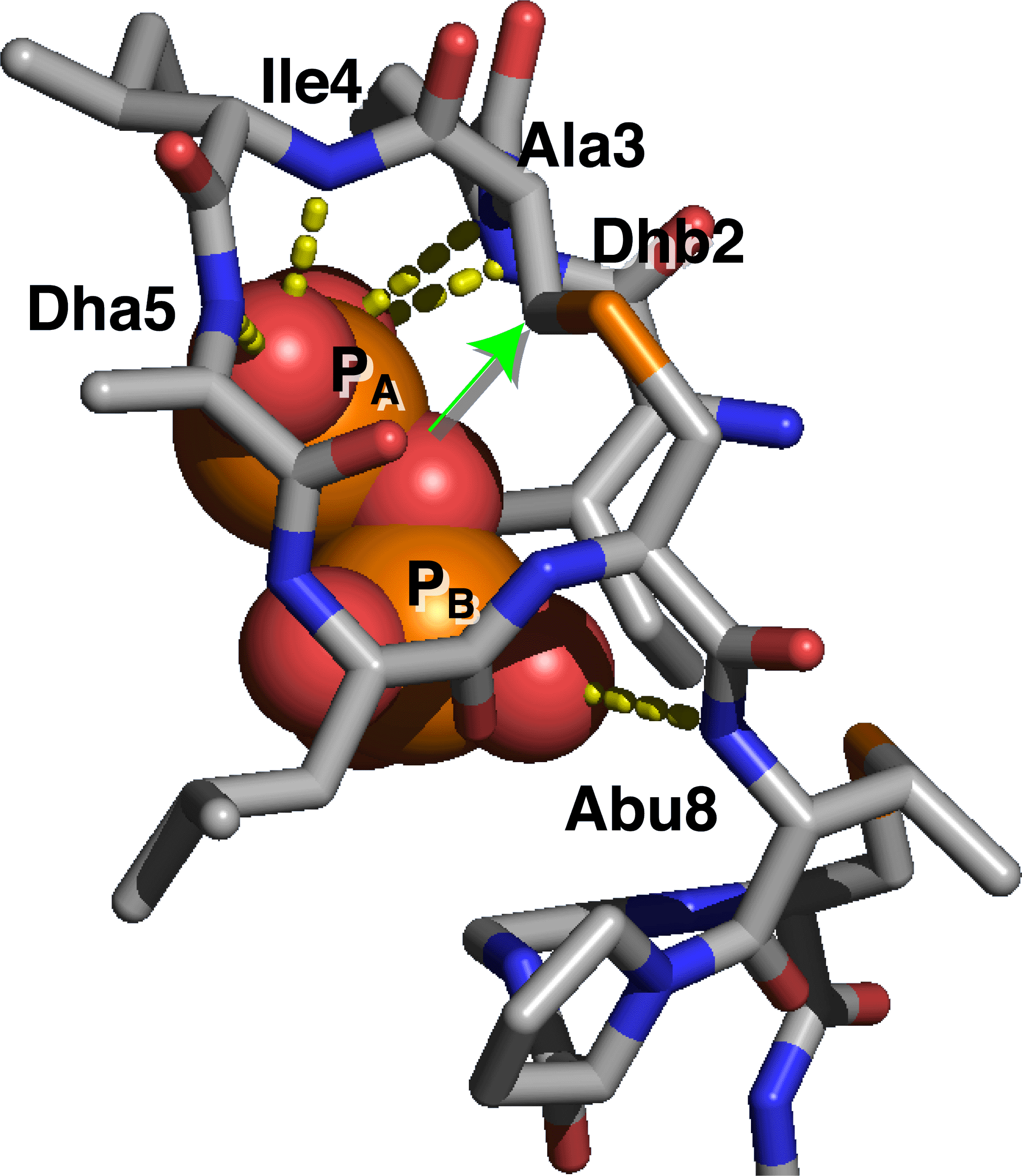
Nisin specifically interacts with Lipid II via its N-terminus. The N-terminus forms a cage-like structure in which the pyrophosphate of Lipid II fits nicely. Interestingly, the pyrophosphate is bound mainly via hydrogen bonds to the backbone NH of nisin. Thus side-chain interactions only play a minor role in the interaction of nisin with Lipid II.
|
|
|
|
 |
|
|
|
 |
|
| Pictures will be placed on the website after they've been published........ |
|
|
|
| Lipid II mediated pore-formation by nisin |
|
| References:
Breukink, E. et al. (1999) Science 286, 2361-2364.
Breukink, E. & de Kruijff, B. (1999) Biochim. Biophys. Acta 1462, 223-234.
Brotz, H. et al. (1998) Mol. Microbiol. 30, 317-327.
Hsu, S.-T.D. et al (2004) Nature Structural and Molecular Biology in press
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Yep, you're right!!!! This should have been micro molar.
Transglycosylation/peptidation. In this step the subunit is transferred to a pre-existing cell wall acceptor. Peptide modifications occur, e.g., addition of the bridge peptide to the lysine (a pentaglycine in case of S.aureus), the terminal D-Ala of the pentapeptide is lost during a trans-peptidation step.
The peptidoglycan polymer of gram-positivebacteria is 15-50 nm thick. One glycan strand may be 10 to 200 disaccharide units long. These strands are crosslinked via a peptide bridge between the C-terminal D-Ala and the w-aminogroup of the L-R3 diamino acid (lysine in the picture).
C-terminal D-Ala-D-Ala sequence recognized by the antibiotic vancomycin.
Step catalysed by MurG (N-acetylglucoseaminyl transferase)
Uridine diphosphate activated GlcNAc
Bactoprenol carrier composed of 11 isoprene subunits (undecaprenol).
Like other pore-forming peptide antibiotics (e.g., magainin) nisin is overall positively charged (net charge of 3.8 at pH 6).
Instead of the more general disulphide bonds, nisin contains thioether bonds that are not reducible.
Nisin contains some unusual amino acids which originate from dehydrated Serine (Dha) or Threonine (Dhb) residues.







